STOPPING COOLING SYSTEM CORROSION
The most common form of destruction for an engine, and one of the oldest and most overlooked problems, is electrolytic metal erosion (EME). Most motorheads have probably never heard of EME, but you all know what the signs are. EME is often mistaken for normal corrosion caused by oxidation in the cooling system or for erosion caused by coolant movement throughout the engine. Evidence of EME can be found virtually anywhere water contacts the above metals, such as around the thermostat housings and water jackets in the cylinder heads and on water-pump gears, heater-hose inlets and outlets, and other aluminum components.
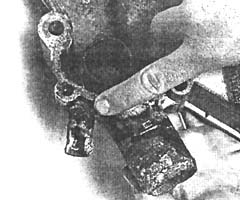

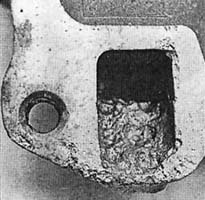
Your radiator acts as a storage battery for the cooling system because you have a tremendous disassociation of metals (such as aluminum, cast iron, copper, and brass), and these metals mix with a mild acidic solution such as the engine coolant. This causes a chemical reaction that removes free electrons from certain active metals such as zinc, magnesium, and aluminum. The loss of electrons, in turn, causes pitting and weakening of the metal. The more acidic the coolant solution is, the greater the EME and the greater the corrosion as a result.
So, if this is such a problem, why isn’t this condition more publicized and how do you rid your cooling system of it? The first question is easily answered. EME isn’t talked about a great deal because it is usually mistaken for normal corrosion or a lack of the proper mixture of coolant and water. The second question is more problematic. You can never entirely rid a cooling system of EME because of the unique situation that calls for the association of different metals. However, there are ways to reduce its effects.
To prevent EME conditions, a metal with a greater positive charge is introduced to the cooling system to be eaten up in lieu of the aluminum components that make up your engine and cooling system, which are more negatively charged. For years, the marine industry has used magnesium to slow the process of EME in cast-iron engine applications. The magnesium is sacrificed to protect more pricey aluminum engine components.
A cooling system running straight water is less susceptible to EME than an ethylene-glycol system because water is much less acidic than typical coolant. However, a straight-water cooling system doesn’t offer the same benefits over the antifreeze system. Many coolant manufacturers are including a small amount of magnesium in their coolant, but it is dissipated rather quickly, forcing you to change the coolant every six to eight months.
Preventing EME in an automotive application is similar to the marine use of sacrificial magnesium. Discovery Development & Engineering offers its new Rad Cap, which is a replacement radiator cap with an attached sacrificial magnesium rod that submerges itself in the cooling system. The magnesium will last roughly 18-36 months, depending upon the vehicle’s usage, before it is completely eaten away. The Rad Cap will have to be replaced every few years, but with a low cost of around $20 at the time of publication, it beats the replacement costs associated with an EME-damaged engine or part. You can get your Rad Cap at: DD&E, Dept. TR, 5236 Pacheco Blvd., Pacheco, CA 94553, (925) 689-6214.
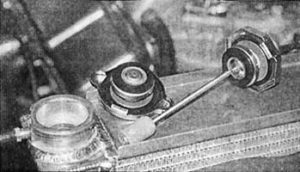
Determining the levels of EME present in the cooling system is very easy. All you really need is an ultrasensitive voltmeter. Simply remove the radiator cap, stick the positive probe into the cooling system, and ground the negative probe to the radiator. With the metal dialed down to the lowest voltage setting, take a reading of the total amount of electrical volts present in the cooling system. The reading should be somewhere between 0.5 volts and 2 volts. Anything higher than 2 volts means the cooling system is highly acidic and should be changed at once to prevent future leaks from appearing.
